Introduction:
It is well stated in the literature that Total Contact
Casting is a standard of care for off-loading of
neuropathic ulcers of the plantar surface. Another
standard of care is to provide a moist wound healing
environment. Evidence to support appropriate
wound dressings under Total Contact Casting is currently unavailable
despite recognition that the wound environment
and thus the amount of drainage changes as the
wound progresses towards healing. This pilot case
series of three patients with plantar based diabetic
neuropathic ulcers uses two dressings, a five layer
silicone foam with absorbent polymer and moisture
retentive backing dressing (SF)* for low to moderate
drainage and a non-adherent super-absorbent
(SAP)† polymer dressing for moderate to high exudate levels.
Clinical Problem:
Many Total Contact Casting kits include a generic, open-cell,
polyurethane foam dressing (OCF). These dressings
do not absorb or retain fluid in a moderate to high
exudate environment which can lead to maceration
and non-healing. Furthermore, due to the high
moisture vapor transmission rate of OCF, wounds
with low exudate may experience desiccation and
result in the development of slough and biofilm.1
Methods:
This pilot case series evaluates the performance of
two dressings under a Total Contact Cast‡. The 1st dressing is a
five-layer silicone foam with super-absorbent
polymer and moisture retentive backing for low to
moderately exuding wounds (SF)*. The 2nd dressing
is a non-adherent absorbent (SAP)† polymer
dressing for moderate to high exudate levels.
A total of 3 Wounds were assessed, cleansed,
debrided if necessary, categorized by exudate level,
and Total Contact Casting was applied for 7 days. The expected
outcomes were to avoid maceration, increase in
healthy granulation/epithelial tissue, and achieve
maximum wear time of a Total Contact Cast.
Results:
All three of the pilot cases healed completely in
acceptable time with no untoward complications.
The important feature was that the changes in the
amounts of drainage which were identified as the
healing progressed were compensated for by
changing the dressings based on the amounts of
exudate identified. This effectively minimized
associated peri-wound maceration, damage to the
increasing granulation tissue and improved
tolerance of their feet to the total contact cast.
Conclusion:
Total Contact Casting kits should consider including dressings for
low-moderate and moderate-high exudate and
cease adding the one-dressing-fits-all generic foam
into the kit. Additionally, although foam dressings
are lumped into single category, their individual
traits such at total volume handling, exudate
retention, and MVTR have erratic variation in
function. The SF in this case series exceeded
expectation and can be a standard of care unless
exudate overwhelms the dressing in the 7 day
expected wear. At that time, SAP should be used in
place of SF to manage high exudate. Our
experience is that this combination of dressings
progresses wounds towards healing, enhances the
clinical benefits and wear time of Total Contact Casting, and
decreases the potential for wound healing
complications.
| Case History 1 45 year old Insulin-dependent diabetic male (IDDM) with a history of poor compliance presented with a plantar ulcer of 2 years duration. He had developed an acute Charcot's Arthropathy, misdiagnosed as osteomyelitis and had surgery to remove "infected" bone. He had ongoing significant drainage with periwound skin maceration and no evidence of healing. Course of Treatment: Aggressive debridement of the ulcer was performed with identified bone at the base of the ulcer. However, further workup did not demonstrate osteomyelitis. He was placed in a Total Contact Cast and SAP used due to concerns about the excessive drainage. With the drainage controlled, maceration resolved and offloading successfully managed, he went on to heal completely. |
|
|
|||||||||||||||||
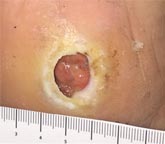
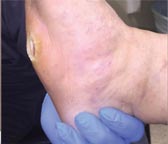
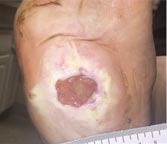
| Case History 3
A 64 year old female with advanced rheumatoid arthritis and foot deformity presented with a 6 month history of a nonhealing ulcer of the right plantar foot. A surgery to correct this had incisional dehiscence. Topical dressings and an offloading boot were previously used unsuccessfully. Course of Treatment An aggressive debridement was performed with no bone exposure noted. Her significant serous drainage was treated with SAP and Total Contact Casting applied weekly for offloading. Over the next several weeks, the drainage decreased and the dressing was changed to SF due to decreasing wound drainage. With the drainage controlled and offloading in place, she went |
 |
 |
 |
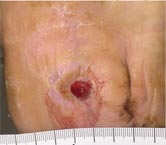
Initial Appearance – 5/17/16 |
Non-healing ulcer, initial appearance Side View |
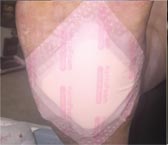
Day 7 – 5/24/16 |
|
 |
 |
 |
|
Day 35 – 6/21/16 |
Weekly SF Application |
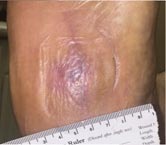
Day 63 – 7/19/16 Complete Healing |